 Understanding the basic principles of dilution refrigeration is essential for operating a ZPC dilution refrigerator effectively. While there are comprehensive guides available on the subject, we provide a condensed overview for quick reference. The first principle involves the cooling of helium isotopes. Helium-4 (4He), the common isotope, liquefies at 4.2 K under standard pressure. Further cooling can be achieved by pumping on it, extracting its latent heat of vaporization. However, the cooling power is limited by the exponentially decreasing vapor pressure of helium. This restricts the achievable temperature to around 0.8 K, realistically reaching 1.2 K in the laboratory.
Understanding the basic principles of dilution refrigeration is essential for operating a ZPC dilution refrigerator effectively. While there are comprehensive guides available on the subject, we provide a condensed overview for quick reference. The first principle involves the cooling of helium isotopes. Helium-4 (4He), the common isotope, liquefies at 4.2 K under standard pressure. Further cooling can be achieved by pumping on it, extracting its latent heat of vaporization. However, the cooling power is limited by the exponentially decreasing vapor pressure of helium. This restricts the achievable temperature to around 0.8 K, realistically reaching 1.2 K in the laboratory.
To achieve even lower temperatures, helium-3 (3He), a rare isotope, is used. Due to its higher vapor pressure compared to helium-4, the base temperature achieved by pumping on liquid 3He is reduced to 0.3 K. However, continuous operation of 3He refrigerators is not practical due to its scarcity. Instead, a small 3He reservoir is condensed by a pumped 4He pot, which is cyclically operated using a closed charcoal sorption pump.
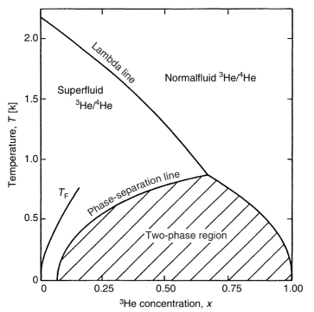 The key to continuous operation below 1 K lies in the phase separation phenomenon of the 3He-4He mixture. By adding 3He to the mixture, the superfluid transition of 4He is suppressed, resulting in two distinct fluids: a nearly pure liquid of 3He (the pure phase) and a dilute solution of 3He in 4He (the dilute phase). Even at very low temperatures, the solubility of 3He remains at around 6.7%, enabling the operation of a dilution refrigerator.
The key to continuous operation below 1 K lies in the phase separation phenomenon of the 3He-4He mixture. By adding 3He to the mixture, the superfluid transition of 4He is suppressed, resulting in two distinct fluids: a nearly pure liquid of 3He (the pure phase) and a dilute solution of 3He in 4He (the dilute phase). Even at very low temperatures, the solubility of 3He remains at around 6.7%, enabling the operation of a dilution refrigerator.
A dilution refrigerator operates by evaporative cooling of the 3He-4He mixture. As the temperature is lowered, the evaporation is predominantly 3He, cooling the liquid into the region of phase separation. The pure phase floats on top of the dilute phase, and the dilute phase in the still undergoes dilution as 3He passes across the phase boundary from the pure side. This process is similar to the transition from liquid to gas phase but with a finite solubility of 3He in the dilute phase.
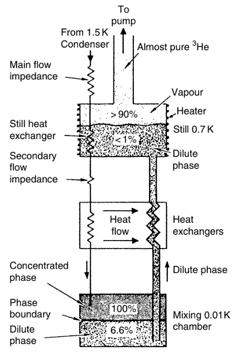 The final step of the dilution refrigerator involves purifying the 3He pumped from the still and returning it to the pure side of the mixing chamber. This is a challenging task in practice, as proper pre-cooling of the pure 3He is necessary along the entire fridge.
The final step of the dilution refrigerator involves purifying the 3He pumped from the still and returning it to the pure side of the mixing chamber. This is a challenging task in practice, as proper pre-cooling of the pure 3He is necessary along the entire fridge.
It's important to note that the cooling power of the dilution refrigerator is proportional to the number of 3He atoms being extracted per unit of time. To maintain optimal performance, additional heat is sometimes added to the still to increase the vapor pressure of 3He and enhance circulation and cooling power.
Zero Point Cryogenics, an Edmonton-based company, specializes in manufacturing ZPC dilution refrigerators for the quantum computing industry. By designing robust and reliable refrigerators that meet industry partners' specific requirements, Zero Point Cryogenics enables customers to focus on developing their quantum technologies without worrying about refrigerator operation.
Figure 1: Phase Diagram. Credit: Frank Pobell (1995); Figure 2: Dilution Refrigerator. Credit: Frank Pobell (1995).
Top Image: Zero Point Cryogenics' Model I Dilution Refrigerator


